Batten Disease Clinical Center of Excellence Program
The BDSRA Foundation is a strong advocate for the provision of specialized, comprehensive clinical care and support for individuals living with Batten disease. The Center of Excellence Program aims to optimize patient outcomes, accelerate research, and improve the quality of life for individuals affected by Batten disease and their families by increasing access to the best possible clinical care and sub-specialty services through a national network of Centers.
Families seeking clinical care for their affected loved ones can be confident that those recognized Centers provide best-in-class care that aligns with the Standards for Designation endorsed by the Batten Disease Centers of Excellence Program.


ABOUT THE CENTER OF EXCELLENCE PROGRAM
Launched in 2024, the official Batten Disease Center of Excellence Program represents the culmination of a shared vision and two years of planning and co-development of a truly patient-centered national CoE model. Under the auspices of the BDSRA Foundation, the Batten Disease CoE Consortium – comprising more than a dozen leading Batten disease clinicians, a parent advocate, and BDSRA Foundation representatives – has proudly co-designed the CoE mission, national model and Standards for Designation.
Program Mission
Through a national network of designated Centers, the CoE Program will:
- Provide the highest level of comprehensive Batten-specific care for patients and their families
- Advance clinical research and drive patient access to emerging therapies
- Provide Batten-specific professional support and education to other healthcare providers, and
- Collaborate with the BDSRA Foundation in their efforts to continually improve the lives of those affected by Batten disease.
Program Pillars & Objectives
ENABLING EQUITABLE ACCESS FOR ALL PATIENTS AND FAMILIES
PILLAR 1 |
PILLAR 2 |
PILLAR 3 |
PILLAR 4 |
|---|---|---|---|
OPTIMAL CARE |
ENHANCING ACCESS TO CARE |
RESEARCH |
EDUCATION & COLLABORATION |
| OBJECTIVE 1 | OBJECTIVE 3 | OBJECTIVE 5 | OBJECTIVE 7 |
| To provide the best possible rigorous and systematic, evidence-based multidisciplinary care and comprehensive expert clinical services for individuals with Batten disease and their families. | To increase patient access to specialized care through a national, geographically distributed network of Centers of Excellence. |
To drive and develop Batten disease research with a focus on advancing treatments through participation in clinical, translational and/or basic research. | To provide peer-to-peer education, support and mentorship. |
| OBJECTIVE 2 | OBJECTIVE 4 | OBJECTIVE 6 | OBJECTIVE 8 |
| To advance knowledge and continuously develop best practice and standards of care for Batten disease, through standardized collection of patient data and assessments. | To complement and support patients’ local medical care teams, particularly for those unable to access Centers of Excellence for regular care. | To actively participate in collaborative research and data sharing. | To ensure the future and sustainability of a national Batten disease Center of Excellence network through training and development of emerging Batten-specialized clinicians with each Center. |
| OBJECTIVE 9 | |||
| To support the mission of the BDSRA Foundation. |
Figure 1. Batten Disease Centers of Excellence Program Pillars and Objectives
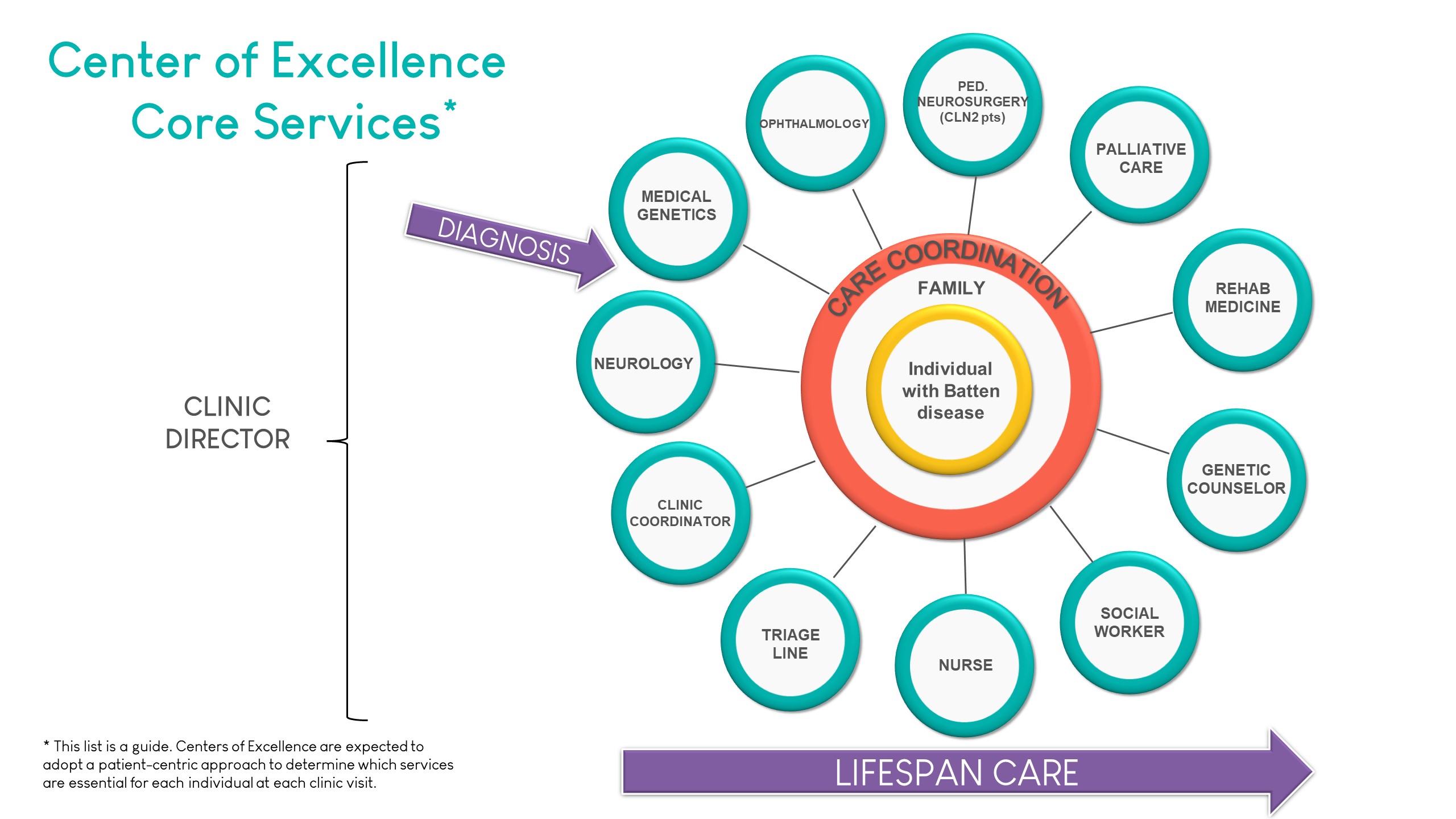
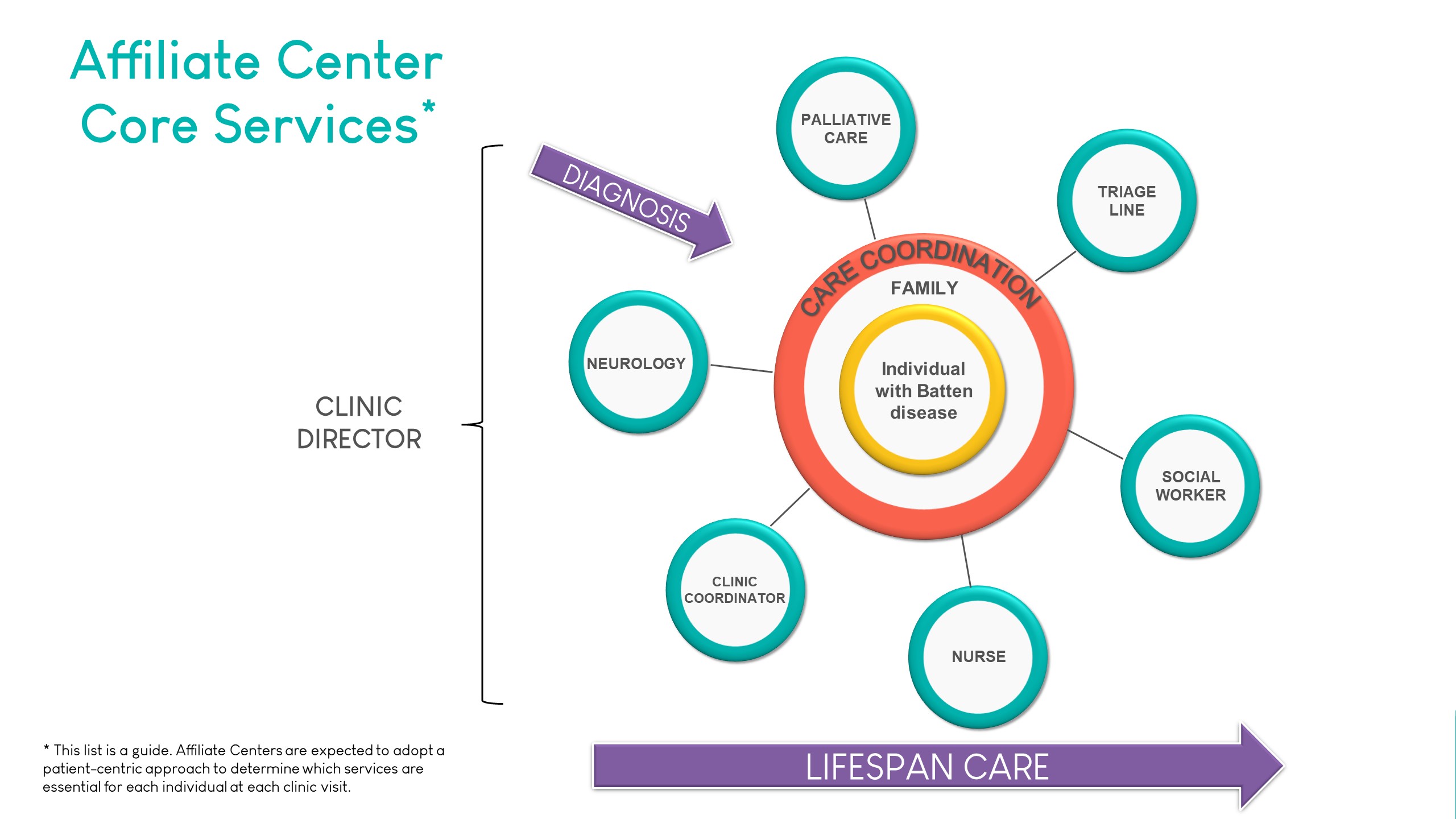
Designations
Requirements for Designation include providing standardized comprehensive clinical services and care in agreement with the Standards for Designation as established and endorsed by the Batten Disease CoE Consortium. All designated Centers of Excellence and Affiliate Centers have met the respective requirements and agree to provide standardized comprehensive clinical care and services.
An important criterium for designation is the provision of the core services required for optimal multidisciplinary care. The list of services below illustrates the range of services typically offered by our Centers.
Who can apply?
Applications for the Center of Excellence Program are open to all clinics in the United States that share BDSRA Foundation’s commitment to high-quality, comprehensive care, advancing clinical research, and being a genuinely collaborative partner within the national Center of Excellence network.
Applications for designation will be considered at any time, however financial support from the BDSRA Foundation will only be offered according to the CoE Grant Cycle. Applicants need to complete the Center of Excellence application process in full, demonstrating that clinical practice is consistent with the Program’s Standards for Designation.
If your center is interested in joining the Batten Disease Center of Excellence Program, please email CoE@bdsrafoundation.org.
ENHANCING CARE, RESEARCH, & CLINICAL TRIALS
By standardizing care at Batten Disease Centers of Excellence (CoEs), many of which serve as major clinical trial sites, we enhance Batten research and clinical trials by reducing variability in care and improving the quality of clinical trial outcome measures. This accelerates the time it takes for therapies to reach those in need.
If you are caring for, or have cared for, a loved one with any form of Batten disease, another way you can help us drive research is by joining the BDSRA Foundation Family Register, which takes just a few minutes to complete. In addition, we encourage all Batten families to consider enrolling in the CoRDS Register (Coordination of Rare Diseases at Sanford). This survey collects more detailed information on your child’s health and medical history and takes around 30 minutes to complete.
Not only will your data help advance research and treatments for Batten disease, but joining these registers will also help you learn about and enroll in actively recruiting clinical trials and research studies.