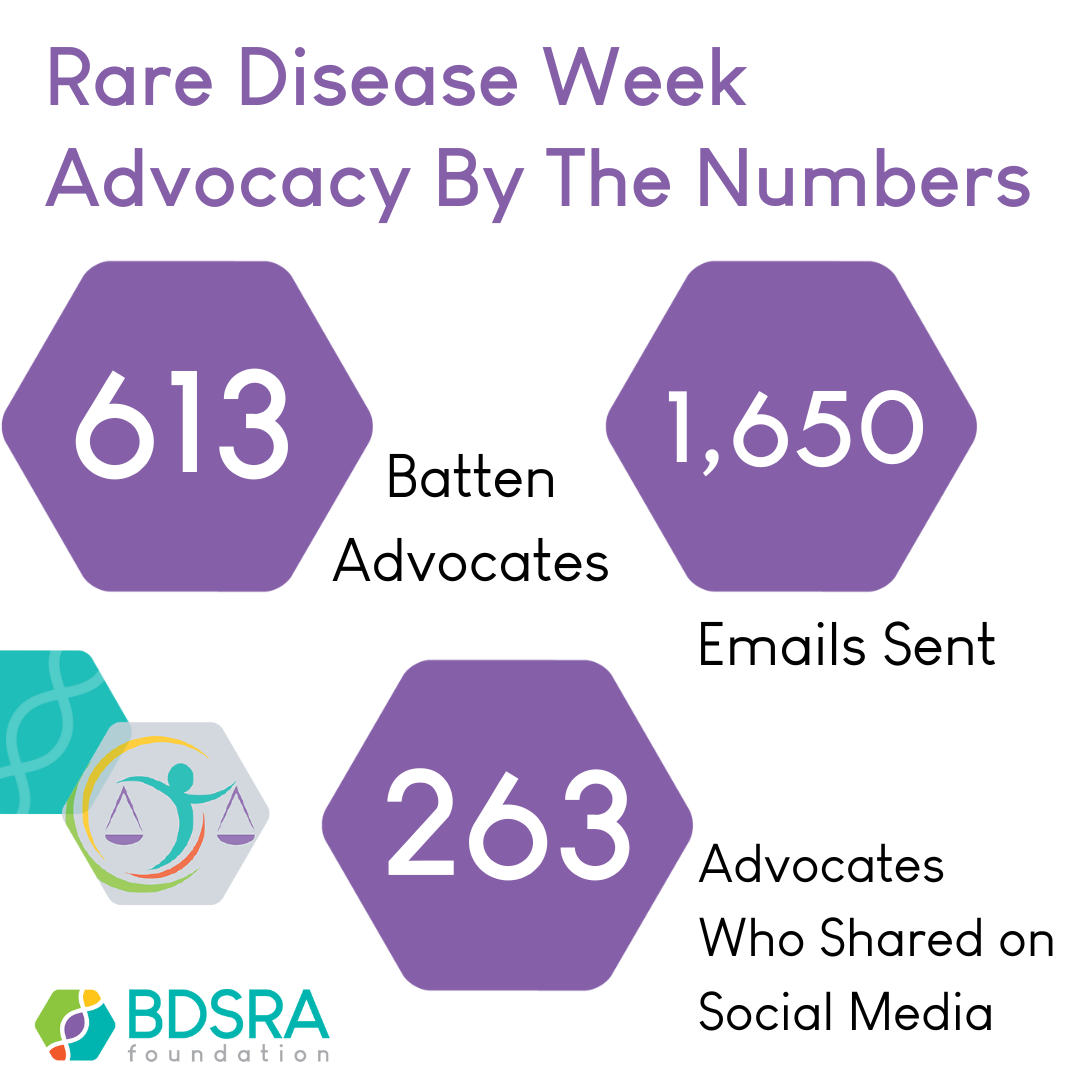
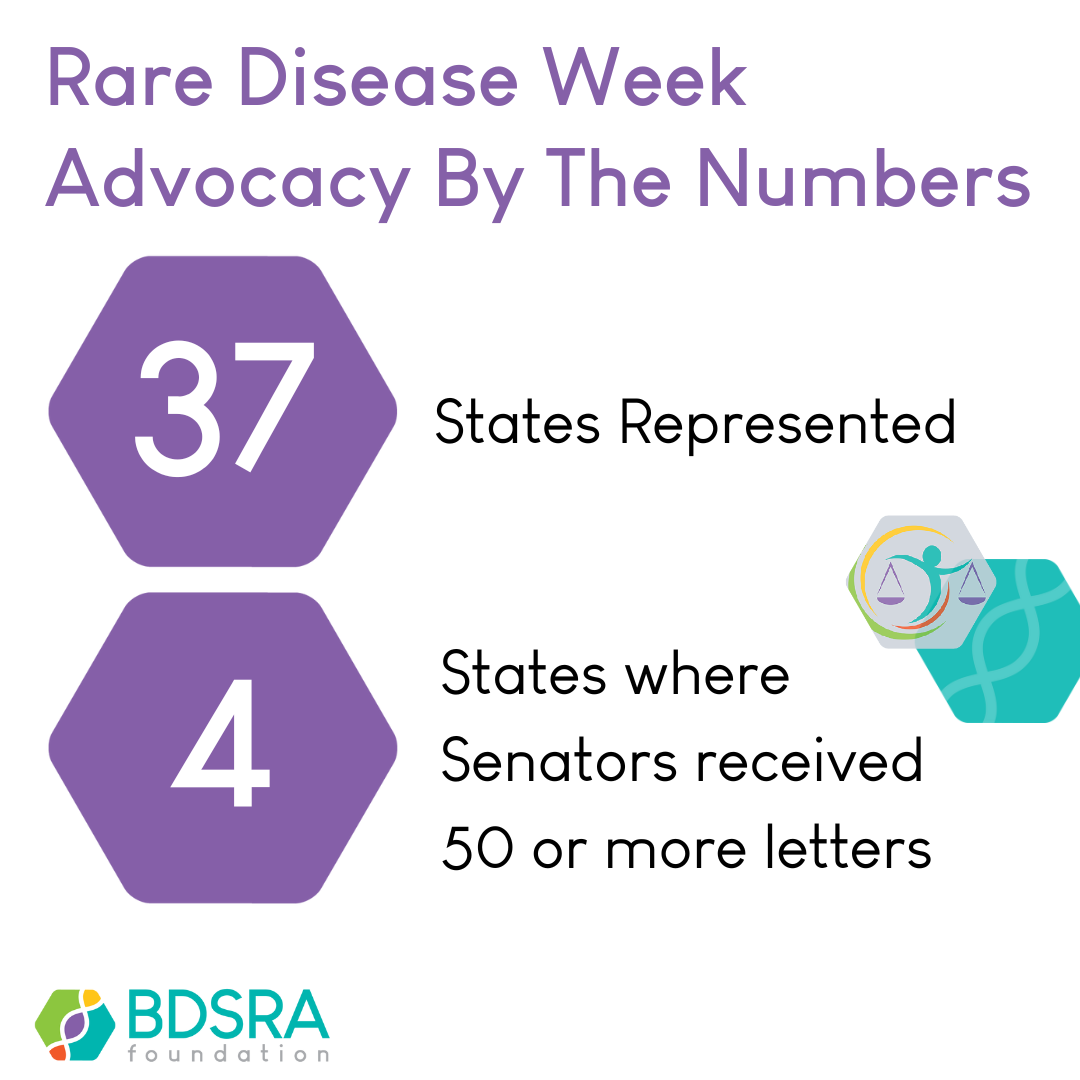
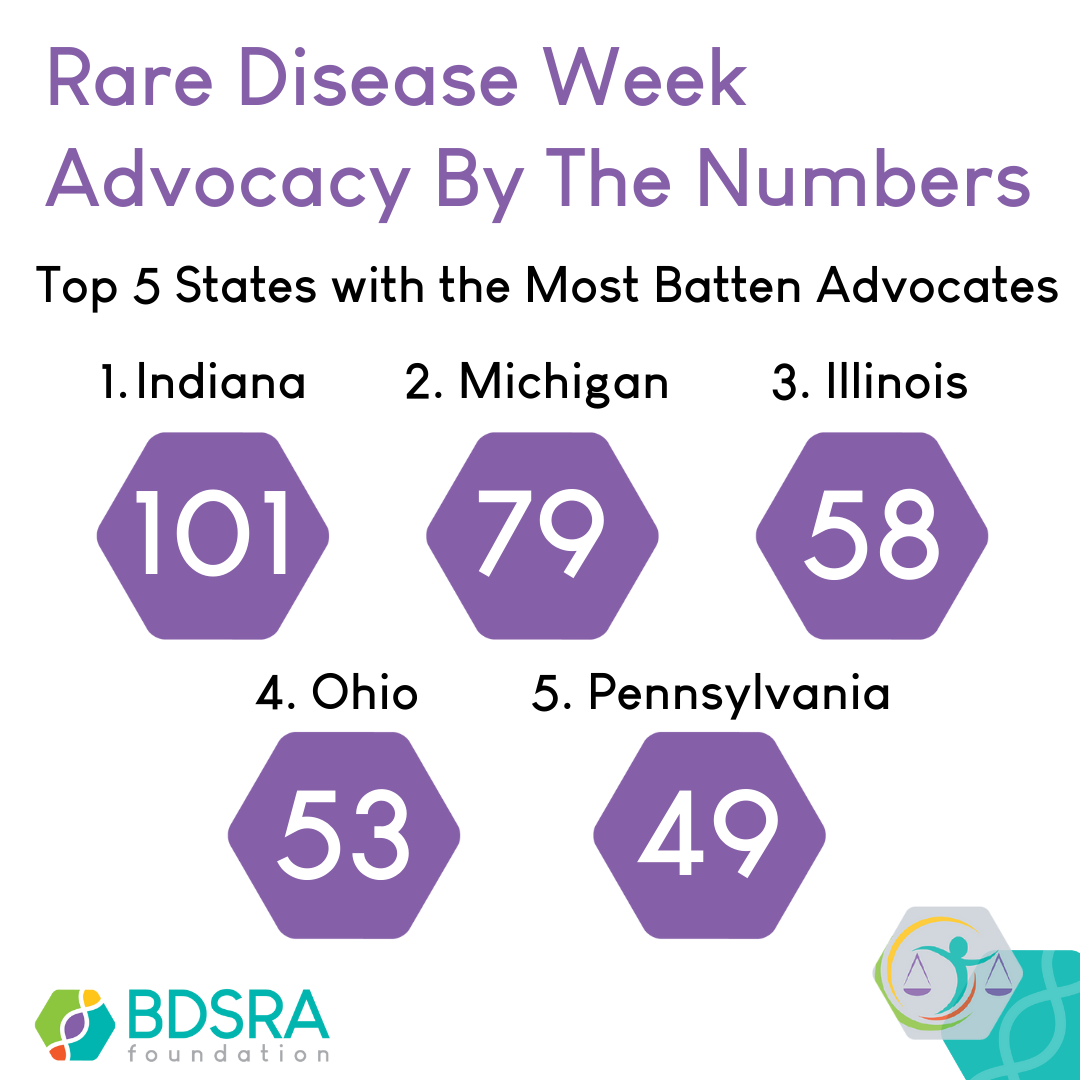
Advocacy Tips from a Batten Father
BDSRA President & CEO Amy Fenton Parker attended Rare Disease Week on Capitol Hill in 2024 and met with staffers representing Ohio legislators. Click here to read the Q&A summary about her experience.
BDSRA Database Manager and rare disease sibling Noah Siedman gives updates on the Rare Disease Day at FDA virtual event and his meeting with BioMarin.
Watch the video below for takeaways from BDSRA staff and a board member regarding Rare Disease Day events.
BDSRA Marketing & PR Coordinator Patrick Kotnik met with two staffers representing Ohio legislators. Watch his summary below!
COLUMN: Rare Disease Week offers a storybook ending and road for optimism
We were witnesses to a storybook ending on a day dedicated to raising awareness and educating others on Rare Disease Day.
Ron Bartek, the Co-Founder and President of Friedreich’s Ataxia Research Alliance (FARA), received word shortly before Rare Disease Day at NIH wrapped up that the FDA approved the first-ever drug for Friedreich’s Ataxia (a rare inherited disease that causes progressive damage to your nervous system and movement problems) called SKYCLARYS for the treatment of people 16 years or older.
Relief, emotion, and catharsis consumed Bartek, who lost his son, Keith, at age 24 due to heart failure from Friedreich’s Ataxia.
Click here to read moreInterested in Advocating?
Feel free to share this BDSRA one-pager in your meetings with state and local representatives!