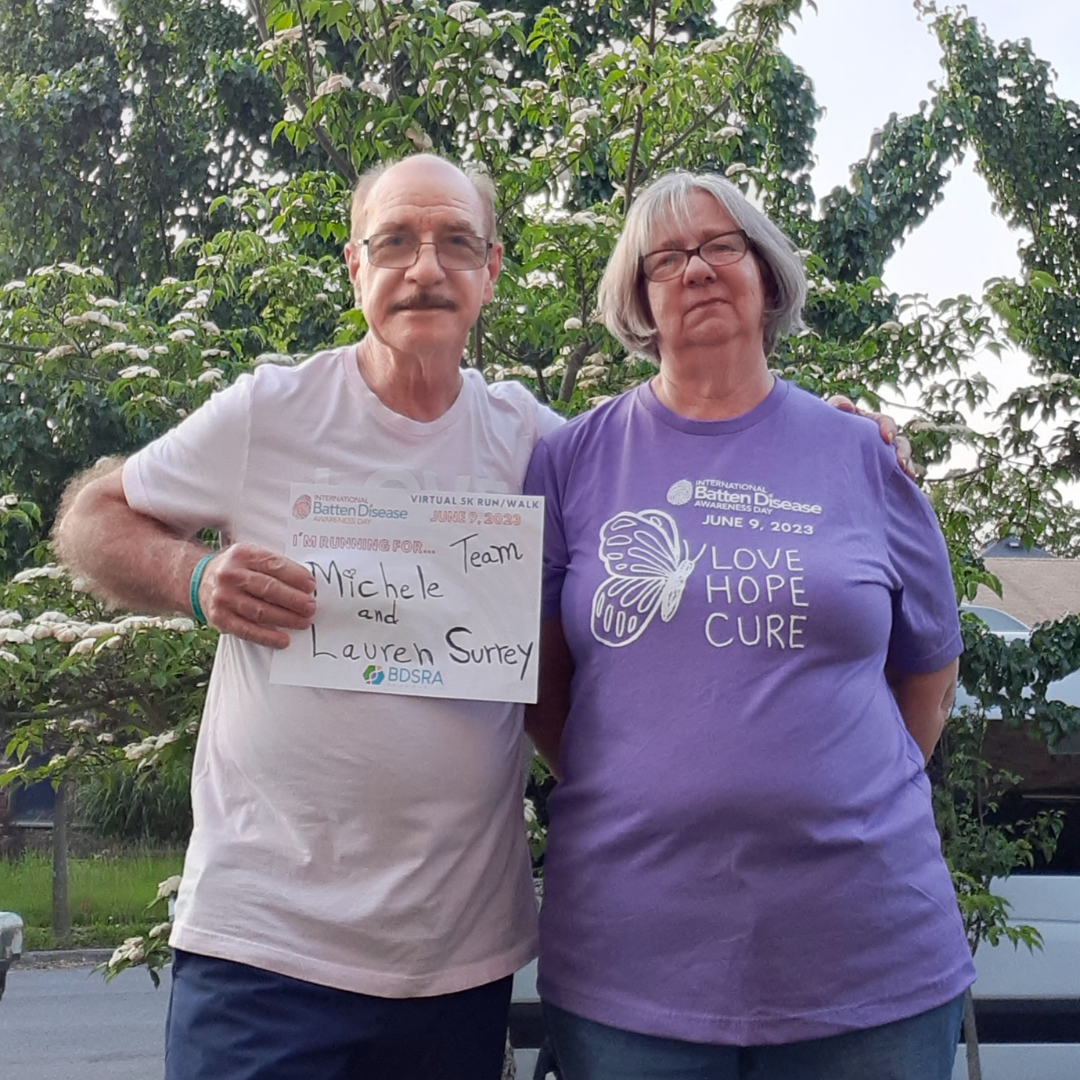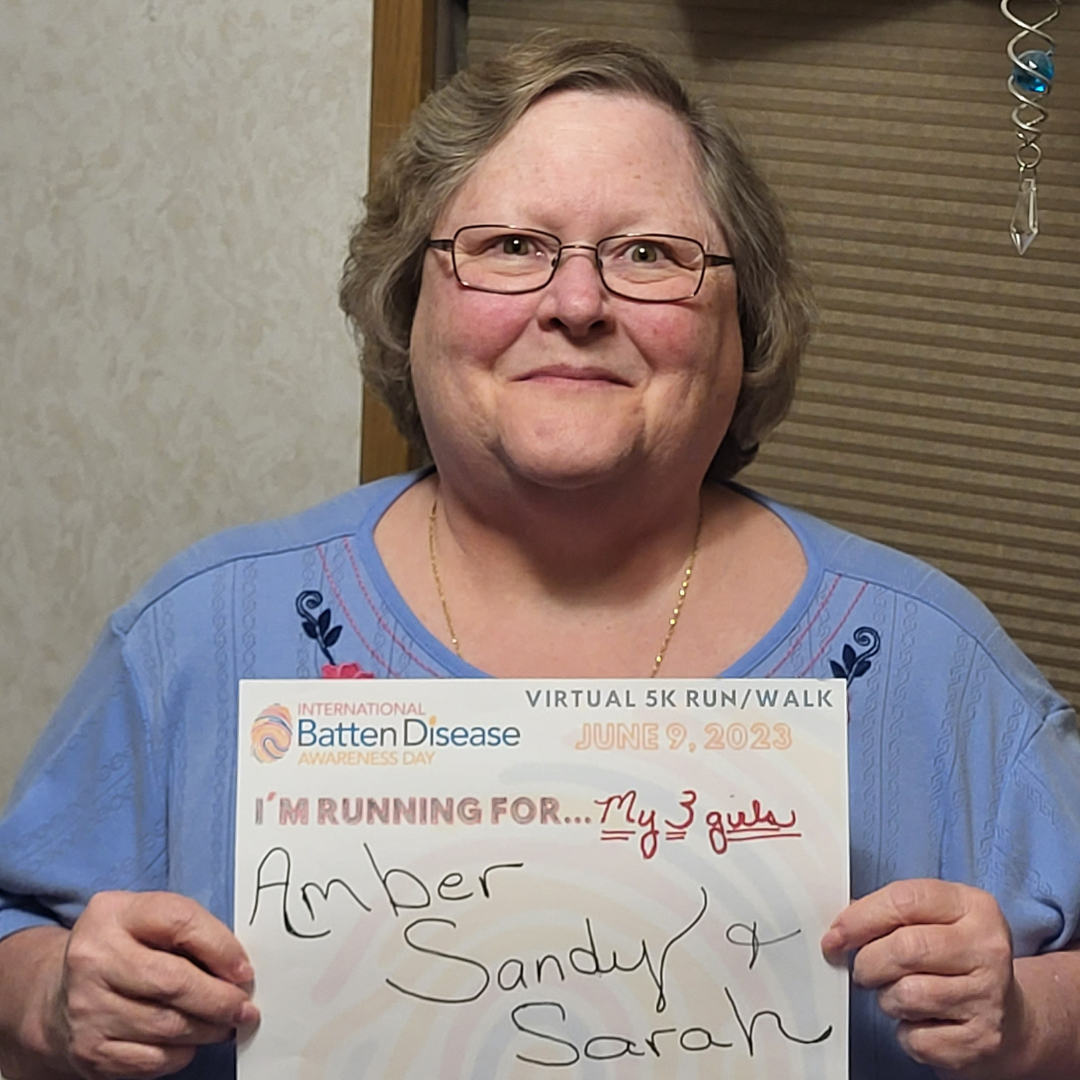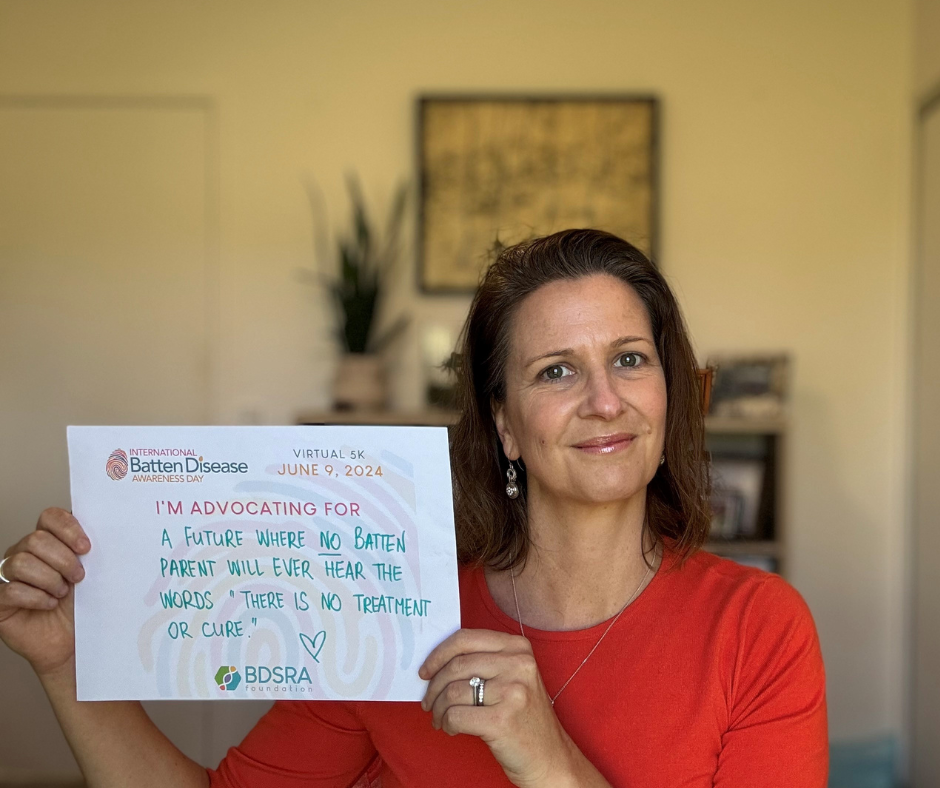Each year BDSRA staff, board members, and parent advocates travel to Washington, D.C. to meet with legislators and appear before committees to push for accelerated drug discovery, increased services for those with rare disease, and improved FDA processes and other initiatives important to our families. Along with the Rare Disease Legislative Advocates, we join with other patient advocacy groups to speak for those who cannot.
BDSRA board, staff, and volunteers have also advocate across state legislatures during Rare Disease Day and Batten Awareness Weekend to raise awareness and increase research related funding for NCL studies. Parents of children with Batten disease have testified to committees in numerous state legislatures on a range of issues, including access to medications, accessibility, and support for services to those caring for patients with life limiting illness.
In 2013, Batten disease advocates appeared on program panels of the National Institutes of Health and National Institute of Medicine to discuss medical and ethical ramifications of gene therapy in rare disease research. In February 2014, our Batten team from BDSRA, Taylor’s Tale and Noah’s Hope visited over 40 Congressional offices on Rare Disease Day to talk about the care needs of Batten children and push for drug discovery.
Parents and board members also have testified before the FDA during public meetings and panels on patient and caregiving challenges for rare disease. Legislative alerts, testimony and panel opportunities, and FDA updates are made available to the Batten community through BDSRA communication channels.
BDSRA actively engages in educational initiatives within the medical community nationally to inform and educate physicians, neurologists, nurses, allied health professionals, genetic counselors and palliative care specialists about Batten disease, the diagnostic challenges, and research and support services available to families.
Resources for rare disease advocacy also include:
June 9th International
Batten Awareness Day
June 9th of every year is designated as International Batten Awareness Day. Families, their advocates, and supporters bring the message of hope and awareness of the disease to thousands of people and inspire charitable gifts to BDSRA. Through social media campaigns, hometown fundraisers, school and church events, and awareness projects, volunteers explain their connection to Batten disease and the importance of raising funds for support and research. BDSRA hosts an annual fundraiser that raises funds to help further our mission.

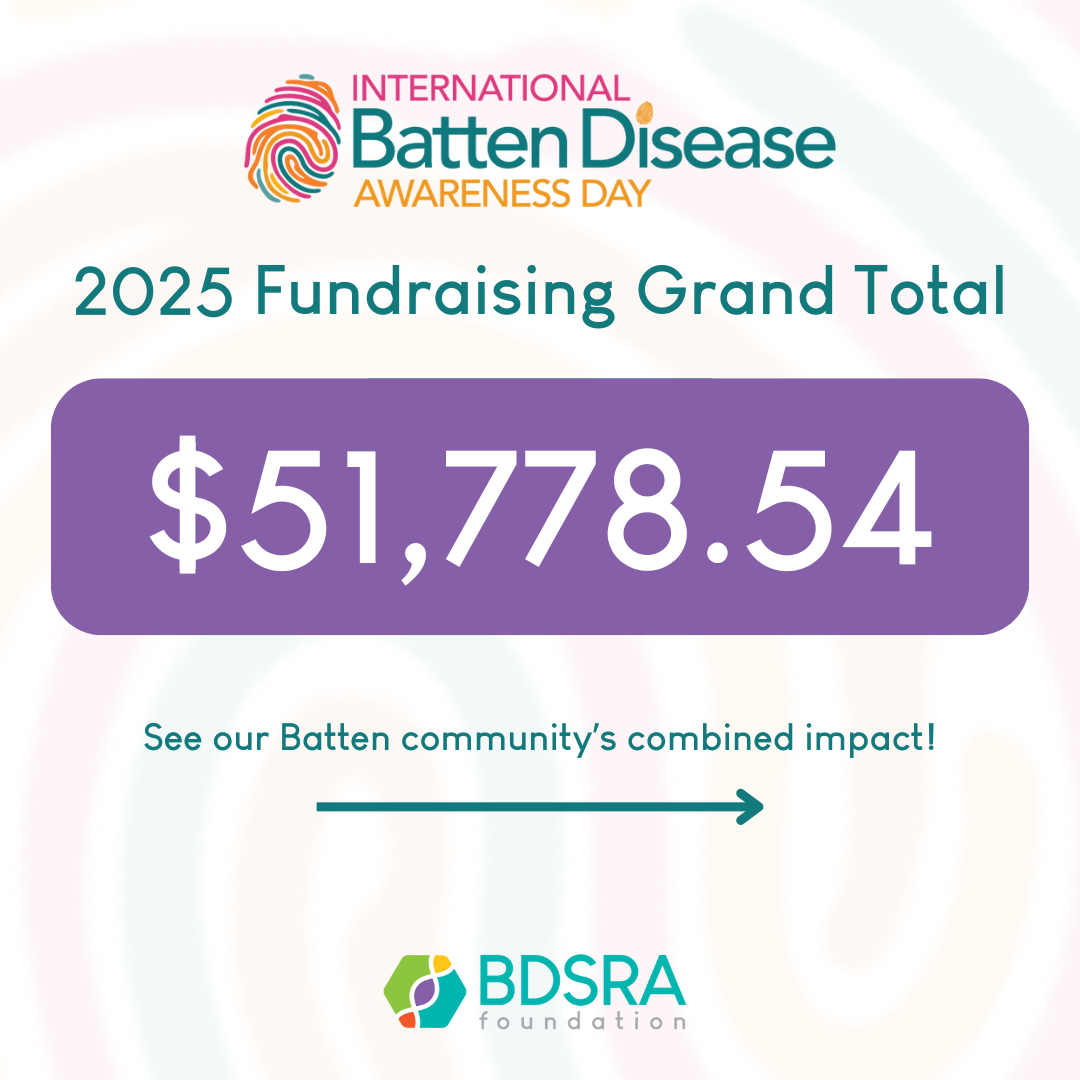
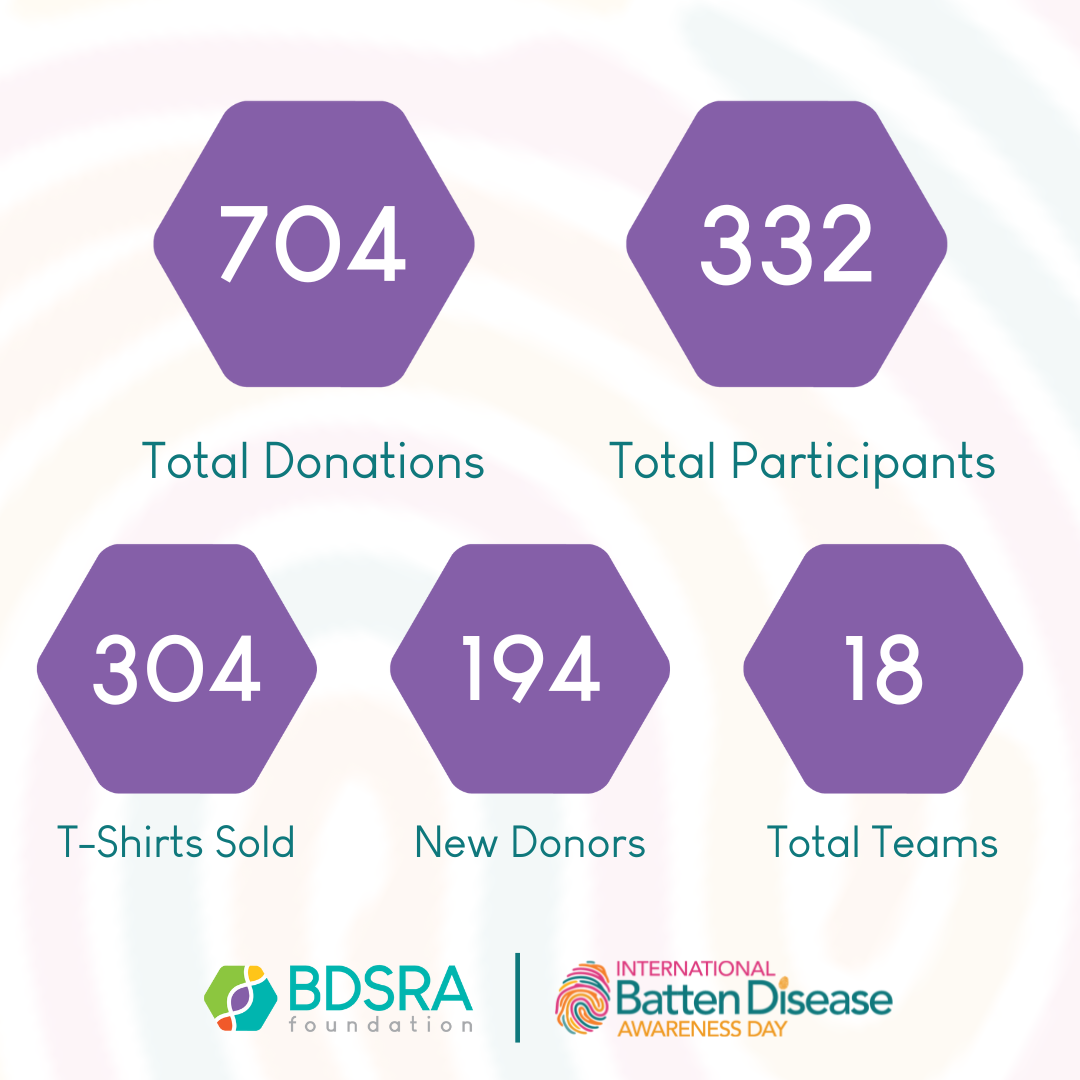
2025 International Batten Disease Awareness Day
A big THANK YOU to all of our donors, Fam Funds, families who hosted fundraising events, and everyone who shared our posts and advocated through our International Batten Disease Awareness Day activities! Your continued gifts, support, and advocacy are crucial so that BDSRA can continue to provide support to families in need, source research for treatments and cures, and advocate for equitable access to healthcare for families. With your continued support, we will accomplish this: https://ow.ly/saSG50Win9R

Recent News
Batten Disease Research Updates with Dr. Ineka Whiteman | February 2026
It's time for Batten disease research updates! BDSRA Foundation's Head of Research & Medical Affairs, Dr. Ineka Whiteman, breaks down Batten disease clinical program updates, research news, and resources in her quarterly column, as [...]
GET INVOLVED: Rare Disease Week 2026
Want to get involved? Visit our website to see all the different ways you can help the Batten disease community and BDSRA, whether it’s fundraising, raising awareness, advocating, or more. Learn More DON'T [...]
Batten Disease: Rare Disease Week 2026
Click below to learn more about what you can do for the Batten disease and rare disease communities this Rare Disease Week! SHOP There are three current shopping fundraisers [...]
Latest Batten Disease and BDSRA Fundraiser & Conference News
Shop to benefit BDSRA and the Batten Community! SHOP NOW: Paper Clouds Apparel will donate 50% of profits from the “Love is Blind” Braille collection to the BDSRA Foundation. This collection features the word [...]
JANUARY 2026 NEWSLETTER | The Illuminator From BDSRA
January 2026 [...]
BDSRA Foundation Announces Boys Town National Research Hospital as New Site in Clinical Centers of Excellence Program; Two Sites Earn CoE Promotions
GAHANNA, OH (January 29, 2026) – The Batten Disease Support, Research, and Advocacy (BDSRA) Foundation has officially announced Boys Town National Research Hospital as the newest site in the Batten Disease Clinical Center of [...]