NCL RESEARCHERS CHALLENGE 2023:
Meet Dr. Joshua Dearborn and the WashU team
As part of this year’s Annual Family Conference program in July, we hosted the inaugural NCL Researchers Challenge. Batten disease researchers from all over the world were invited to enter a “three-minute thesis” style video presentation, summarizing their team’s main research approach, latest findings, and what the significance of these findings is for our Batten community. Conference registrants were invited to view and vote for their favorites. It was so wonderful to see the level of interest and engagement from both the research and family communities!
Congratulations to Dr. Joshua T. Dearborn, Instructor of Medicine, Washington University School of Medicine in St Louis (WUSTL), who was awarded second place for his presentation: Medical Marijuana and Batten Disease. We recently interviewed Dr. Dearborn to learn more about this exciting research.

Dr. Joshua T. Dearborn
1. Please tell us a bit about your team at WUSTL and the research carried out in your lab.
Our team that works on the NCL-related seizure projects is made up of myself, Dr. Mark Sands, Dr. Michael Wong, Nick Rensing, Dr. Keigo Takahashi, and Dr. Jonathan Cooper. I’ve worked as part of a team with Dr. Mark Sands since 2014. Generally speaking, we use mouse models of lysosomal storage disorders to do two things: understand the nature and progression of these diseases and try to correct disease-related pathology and symptoms. We have studied a mouse model of CLN1 for many years, applying such treatments as gene therapy, enzyme replacement, and bone marrow transplant. Recently, I’ve undertaken the study of a CLN2 mouse model.
2. How did you first become interested in the study of medical marijuana in Batten disease?
Two events led to the onset of this research, both occurring some six or seven years ago. First, Mark Sands and I discussed a recent episode of the television show Dateline which profiled a group of brothers in Colorado who were growing a strain of cannabis plant with the specific aim to aid a child with epilepsy. Second, at a BDSRA Family Conference, former BDSRA Executive Director Margie Frazier presented a survey taken from Batten caregivers, asking essentially, “Other than finding a cure, what is something that you would find most beneficial if it could be improved?” The most popular answer was “seizures”. Dr. Sands and I took this as serendipity, so we embarked on getting a DEA Schedule 1 license and secured pilot funding from the BDSRA (including support from BDSRA Australia and Drew’s Hope).
3. What have been some of the most interesting findings in this field of research?
I’ve quite enjoyed learning from NCL patients and caregivers their stories of trying out medical marijuana; the takeaway seems to be that it helps some people, but just doesn’t help others. What I find interesting and frustrating, is that we still don’t know why the stuff works, when it does work. Marijuana contains over 400 components we could study, many of which are unique to the cannabis plant, but the one that has been studied the most in terms of potential therapeutic benefit is cannabidiol (CBD), and that’s what I’ve applied to both CLN1 and CLN2 mice. I think it’s interesting that cannabidiol appears to have a positive effect when a mouse is GIVEN a seizure by some external method like a drug or electricity, but we have very little research telling us what cannabidiol will do for seizures that occur as a result of progressive pathology, like that seen in the NCLs. There is still a lot of room for this research, and we’re trying to fill in that space with a specific eye toward the NCLs.

CLN2-affected mice are fed cannabidiol (CBD) incorporated into artificially sweetened and flavored gelatin cubes and monitored for seizure activity.
4. Where to next for this research?
I’m wrapping up my study of CLN2 mice that have been treated long-term with cannabidiol alone; the resulting scientific manuscript will hopefully be available in early 2024. In speaking with clinicians, a pattern seems to be emerging that medical marijuana works best in combination with other drugs; in other words, it may offer limited benefit if used on its own. Specifically, patients that benefit from cannabidiol therapy are often found to be taking an anti-seizure drug called clobazam. I think our next step is to use this combination in the CLN1 and/or CLN2 mouse, asking whether it can knock down the seizures in these mice and hoping to reveal any changes in disease pathology that might explain these effects.
5. Families play an important role in helping advance NCL research in all kinds of ways – even for basic (laboratory-based) work like this. Can you tell us about the role families play in your research, specifically?
To put it simply, I’m conducting this research as a direct result of the BDSRA survey I mentioned in an earlier answer. Caregivers and families told the BDSRA what they would like targeted the most, a group of us at WUSTL were in a position to have a go at it, and that’s what we’ve been doing. As a result of this research, I’ve had conversations with families who generously share their thoughts and experiences with medical marijuana, and that has given me insight into treating and evaluating seizures in CLN2 mice. I also have one family in particular to thank for funding much of this CLN2 research; Dr. Sandra and Stephen Lehrman have generously supported me in carrying out these studies.
6. Finally, for a bit of fun, what does your research team like to do when you’re not busy in the lab?
We are spread across various departments here at Washington University and work in different buildings, but we try to see each other when we can. Outside of the lab, many of us like to visit local breweries, our incredible St. Louis Zoo, and sporting events. We also relish the opportunity to travel to scientific conferences, allowing us to explore new cities and meet up with families and other researchers.
Thank you so much, Dr Dearborn. We look forward to hearing further developments on this project in the future and will look out for your publication in 2024!
CENTERS OF EXCELLENCE PROGRAM: Inaugural Meeting – Sioux Falls, SD
On November 10, members of the Batten clinical community, board members, and BDSRA staff came together from Australia to North Carolina, to reimagine a national Batten Center of Excellence (CoE) program.

Attendees (in-person and virtually) came together in Sioux Falls, South Dakota for the inaugural Batten Center of Excellence Program meeting.
Following our call for applications in September, we were beyond delighted and humbled to have received applications from 11 institutions from around the U.S., seeking to join our CoE Program. It was a highly productive day, with focused, energized discussions, a genuinely collaborative vibe, and the group sharing a unified vision for a program that aims to optimize patient outcomes and quality of life for individuals affected by Batten disease and their families. This first class of applicants will work together under the auspices of the BDSRA Foundation, to co-design and implement the best possible CoE model, that will increase patient access to the best possible standardized, evidence-based multidisciplinary care and clinical services through a national network of Centers.
Meetings will be held regularly from now until July 2024, to develop and implement the Centers of Excellence Program, according to the timeline below. Stay tuned to our social media and The Illuminator for further updates in the coming months.
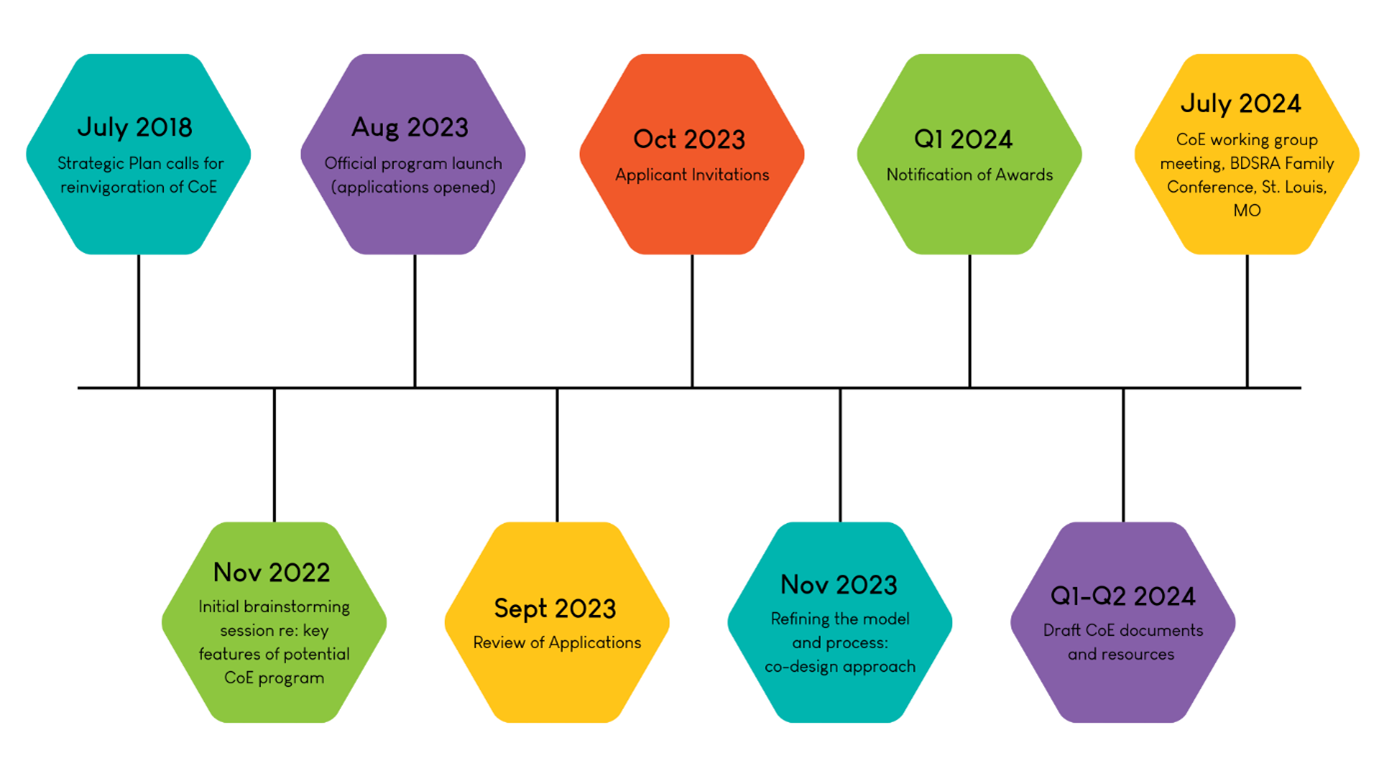
Centers of Excellence Program: Development and Implementation Timeline
Our sincerest thanks to all our applicants and meeting attendees. We are grateful for your care, expertise, and dedication to our Batten families and community.
CLINICAL PROGRAM UPDATES
Clinical Trial Tracker
Have you seen our NEW chart tracking clinical trials for Batten disease? Learn more with this Toolbox Tuesday video. View the chart by clicking here.
REGENXBIO CLN2 Gene Therapy Program Update
On November 8, REGENXBIO announced in a press release its intention to halt the development of potential AAV gene therapies for multiple programs, including the RGX-181 and RGX-381 programs for the treatment of CNS and ocular manifestations of CLN2 disease, respectively.
We worked swiftly with our global patient advocacy partners to issue a joint statement on this matter, a copy of which can be read here.
REGENXBIO has assured us that it will be actively exploring a range of partnership opportunities to enable the continuing development of its CLN2 gene therapy programs. Through ongoing discussions this month, including with President and CEO Ken Mills, we have been encouraged to hear REGENXBIO is indeed taking proactive and practical steps in seeking solutions for the programs – and we will continue to support and urge them for as long as it takes.
On Thursday, November 30, the BDSRA Foundation hosted an open webinar to share the latest updates on REGENXBIO’s CLN2 disease programs, followed by a Q&A. You can watch a recording of this event here.
RESEARCH OPPORTUNITIES
SPEECH & LANGUAGE STUDY – CLN2 and CLN3 Batten disease
WE STILL NEED YOUR HELP!
Thank you to all those families who have already signed up and participated in the world-first research study into the characterization of speech and language in individuals with Batten disease (CLN2 and CLN3 disease).
To complete the project, the research team is seeking a few more participants and they would be grateful for your help.
Participation is open to individuals worldwide who are:
– Affected by CLN3 or CLN2 Batten disease
– Age 6 months and older
– Verbal or non-verbal
Bereaved caregivers are also invited to take part in this study.
To find out more about the project and how to get involved, download the flyer by clicking here or contact the team at Murdoch Children’s Research Institute, Melbourne Australia at geneticsofspeech@mcri.edu.au
USING AI TO IDENTIFY GENETIC CONDITIONS – Calling all NCL families
In one paragraph, how would you describe your child’s condition?
The National Human Genome Research Institute (NHGRI), part of the National Institutes of Health (NIH), develops and studies computer-based technologies to better understand medical conditions that have known or suspected genetic causes.
The team is currently studying how different artificial intelligence (AI) models, including public models like ChatGPT, can identify genetic conditions. These models are a growing part of healthcare, and this project seeks to compare how well different models work.
For this study, they are collecting short descriptions from people with genetic conditions (or their carers) so that these different models can be tested. Participants are asked to send one paragraph describing the condition (eg. Batten disease, and subtype) in their child. Please use whatever description is most natural for you. That is, use your own words in the way you might describe the condition to a clinician meeting you for the first time. All contributions will be de-identified.
A copy of the information leaflet can be downloaded here.
Please send descriptions to the NIH project team by emailing medicalgenomicsunit@nih.gov.
For any inquiries, please feel free to reach out to the above email or contact Dr. Ineka Whiteman at research@bdsraaustralia.org.
FAMILY REGISTER
Have you joined the Register yet?
The BDSRA Foundation Family Register is a vital tool that enables us to keep you informed of ongoing Batten disease research, including future clinical research opportunities.
The Register also enables the BDSRA Foundation to better understand the prevalence of Batten disease, including the different subtypes and geographical locations. This helps us tailor our education and support activities according to the needs of our families. The Register is open to all current and bereaved families.
The information collected in this form is kept STRICTLY CONFIDENTIAL. Your involvement in this survey is entirely voluntary, and you may request to be removed from the list at any time. The form takes just a few minutes to complete and can be accessed by clicking here.
Thank you for participating in this important initiative!
PUBLICATION ABSTRACTS – November
Neuronal progenitor cells-based metabolomics study reveals dysregulated lipid metabolism and identifies putative biomarkers for CLN6 disease
Rus CM, Polla DL, Di Bucchianico S, Fischer S, Hartkamp J, Hartmann G, Alpagu Y, Cozma C, Zimmermann R, Bauer P. Sci Rep. 2023 Oct 29;13(1):18550.
Abstract
Neuronal ceroid lipofuscinosis 6 (CLN6) is a rare and fatal autosomal recessive disease primarily affecting the nervous system in children. It is caused by a pathogenic mutation in the CLN6 gene for which no therapy is available. Employing an untargeted metabolomics approach, we analyzed the metabolic changes in CLN6 subjects to see if this system could potentially yield biomarkers for diagnosis and monitoring disease progression. Neuronal-like cells were derived from human fibroblast lines from CLN6-affected subjects (n = 3) and controls (wild type, n = 3). These were used to assess the potential of a neuronal-like cell-based metabolomics approach to identify CLN6 distinctive and specific biomarkers. The most impacted metabolic profile is associated with sphingolipids, glycerophospholipids metabolism, and calcium signaling. Over 2,700 spectral features were screened, and fifteen metabolites were identified that differed significantly between both groups, including the sphingolipids C16 GlcCer, C24 GlcCer, C24:1 GlcCer and glycerophospholipids PG 40:6 and PG 40:7. Of note, these fifteen metabolites were downregulated in the CLN6 disease group. This study is the first to analyze the metabolome of neuronal-like cells with a pathogenic mutation in the CLN6 gene and to provide insights into their metabolomic alterations. This could allow for the development of novel biomarkers for monitoring CLN6 disease.
Read the full article here.
Efficacy of dual intracerebroventricular and intravitreal CLN5 gene therapy in sheep prompts the first clinical trial to treat CLN5 Batten disease
Murray SJ, Wellby MP, Barrell GK, Russell KN, Deane AR, Wynyard JR, Gray SJ, Palmer DN, Mitchell NL. Front Pharmacol. 2023 Oct 24;14:1212235.
Abstract
Mutations in the CLN5 gene cause the fatal, pediatric, neurodegenerative disease CLN5 neuronal ceroid lipofuscinosis. Affected children suffer progressive neuronal loss, visual failure, and premature death. Presently there is no treatment. This study evaluated dual intracerebroventricular (ICV) and intravitreal (IVT) administration of a self-complementary adeno-associated viral vector encoding ovine CLN5 (scAAV9/oCLN5) into CLN5-affected sheep (CLN5-/-) at various disease stages. CLN5 disease progression was slowed in pre-symptomatic sheep who received a moderate dose of scAAV9/oCLN5, whilst a higher ICV dose treatment in early and advanced symptomatic animals delayed or halted disease progression. Intracranial (brain) volume loss was attenuated in all treatment cohorts, and visual function was also sustained in both the early and advanced symptomatic treated sheep over the 24-month duration of the study. Robust CLN5 protein expression was detected throughout the brain and spinal cord, and improvements in central nervous system and retinal disease correlates were observed. These findings hold translational promise for extending and improving the quality of life in both pre-symptomatic and symptomatic CLN5 patients and prompted the initiation of the first in-human Phase I/II clinical trial testing ICV/IVT administration of scAAV9 encoding human CLN5 (https://clinicaltrials.gov/study/NCT05228145).
Read the full article here.
Integrative human and murine multi-omics: Highlighting shared biomarkers in the neuronal ceroid lipofuscinoses
N. Gammaldi, F. Pezzini, E. Michelucci, N. Di Giorgi, A. Simonati, S. Rocchiccioli, F.M. Santorelli, S. Doccini. Neurobiology of Disease. 2023, 189: 106349
Abstract
Neuronal ceroid lipofuscinosis (NCL) is a group of neurodegenerative disorders whose molecular mechanisms remain largely unknown. Omics approaches are among the methods that generate new information on modifying factors and molecular signatures. Moreover, omics data integration can address the need to progressively expand knowledge around the disease and pinpoint specific proteins to promote as candidate biomarkers. In this work, we integrated a total of 62 proteomic and transcriptomic datasets originating from humans and mice, employing a new approach able to define dysregulated processes across species, stages, and NCL forms. Moreover, we selected a pool of differentially expressed proteins and genes as species- and form-related biomarkers of disease status/progression and evaluated local and spatial differences in most affected brain regions. Our results offer promising targets for potential new therapeutic strategies and reinforce the hypothesis of a connection between NCLs and other forms of dementia, particularly Alzheimer’s disease.
Read the full article here.
Language Delay in Patients with CLN2 Disease: Could It Support Earlier Diagnosis?
Nickel M, Gissen P, Greenaway R, Cappelletti S, Hamborg C, Ragni B, Ribitzki T, Schulz A, Tondo I, Specchio N. Neuropediatrics. 2023 Dec;54(6):402-406.
Abstract
Neuronal ceroid lipofuscinosis type 2 (CLN2 disease) is a rare pediatric disorder associated with rapid neurodegeneration and premature death in adolescence. An effective enzyme replacement therapy (cerliponase alfa) has been approved that can reduce this predictable neurological decline. The nonspecific early symptoms of CLN2 disease frequently delay diagnosis and appropriate management. Seizures are generally recognized as the first presenting symptom of CLN2 disease, but emerging data show that language delay may precede this. An improved understanding of language deficits in the earliest stage of CLN2 disease may support the early identification of patients. In this article, CLN2 disease experts examine how language development is affected by CLN2 disease in their clinical practices. The authors’ experiences highlighted the timings of first words and first use of sentences, and language stagnation as key features of language deficits in CLN2 disease, and how deficits in language may be an earlier sign of the disease than seizures. Potential challenges in identifying early language deficits include assessing patients with other complex needs and recognizing that a child’s language abilities are not within normal parameters given the variability of language development in young children. CLN2 disease should be considered in children presenting with language delay and/or seizures to facilitate earlier diagnosis and access to treatment that can significantly reduce morbidity.
Read the full article here.
Acidified drinking water improves motor function, prevents tremors and changes disease trajectory in Cln2R207X mice, a model of late infantile Batten disease
Kovács AD, Gonzalez Hernandez JL, Pearce DA*. Sci Rep. 2023 Nov 6;13(1):19229.
*BDSRA Foundation Board Member, Dr. David Pearce
Abstract
Batten disease is a group of mostly pediatric neurodegenerative lysosomal storage disorders caused by mutations in the CLN1-14 genes. We have recently shown that acidified drinking water attenuated neuropathological changes and improved motor function in the Cln1R151X and Cln3-/- mouse models of infantile CLN1 and juvenile CLN3 diseases. Here we tested if acidified drinking water has beneficial effects in Cln2R207X mice, a nonsense mutant model of late infantile CLN2 disease. Cln2R207X mice have motor deficits, muscle weakness, develop tremors, and die prematurely between 4 and 6 months of age. Acidified water administered to Cln2R207X male mice from postnatal day 21 significantly improved motor function, restored muscle strength, and prevented tremors as measured at 3 months of age. Acidified drinking water also changed disease trajectory, slightly delaying the death of Cln2R207X males and females. The gut microbiota compositions of Cln2R207X and wild-type male mice were markedly different and acidified drinking water significantly altered the gut microbiota of Cln2R207X mice. This suggests that gut bacteria might contribute to the beneficial effects of acidified drinking water. Our study demonstrates that drinking water is a major environmental factor that can alter disease phenotypes and disease progression in rodent disease models.
Read the full article here.



