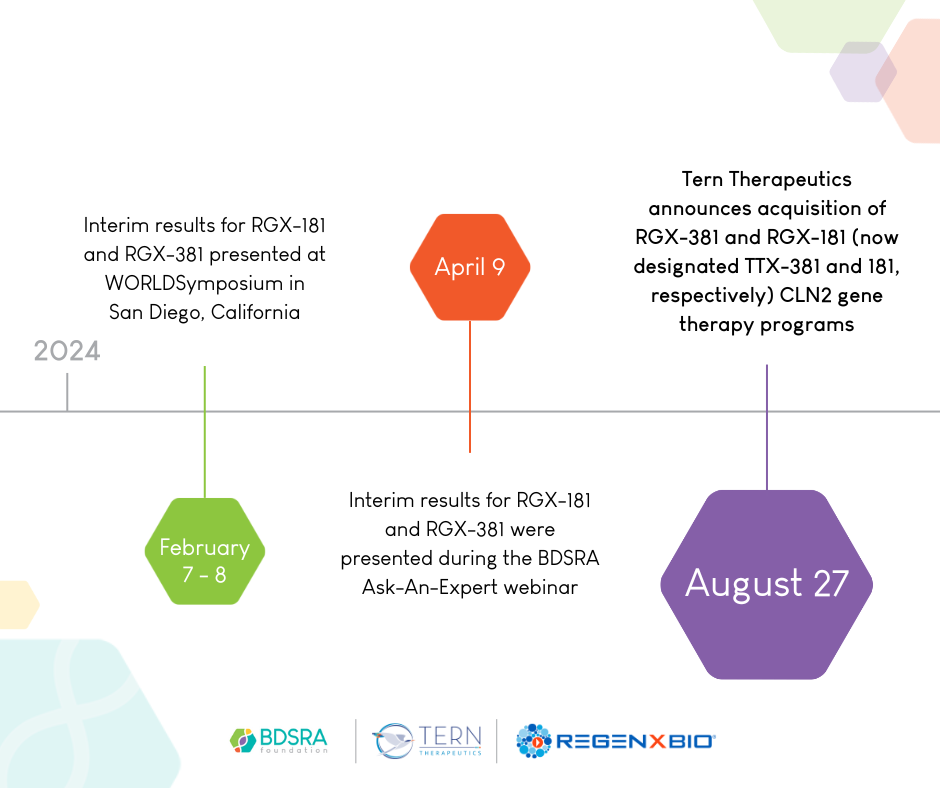
Tern Therapeutics has announced its launch and entered into a global licensing agreement with REGENXBIO for RGX-381 and RGX-181 (now designated TTX-381 and 181, respectively) to form its initial therapeutic pipeline. TTX-381 and TTX-181 are novel one-time gene therapy products being developed for CLN2 Batten disease.
Read Tern’s full press release, which includes statements from BDSRA’s Amy Fenton Parker and Dr. Ineka Whiteman, and REGENXBIO’s letter to the Batten community.
Hear from BDSRA Head of Research & Medical Affairs Dr. Ineka Whiteman
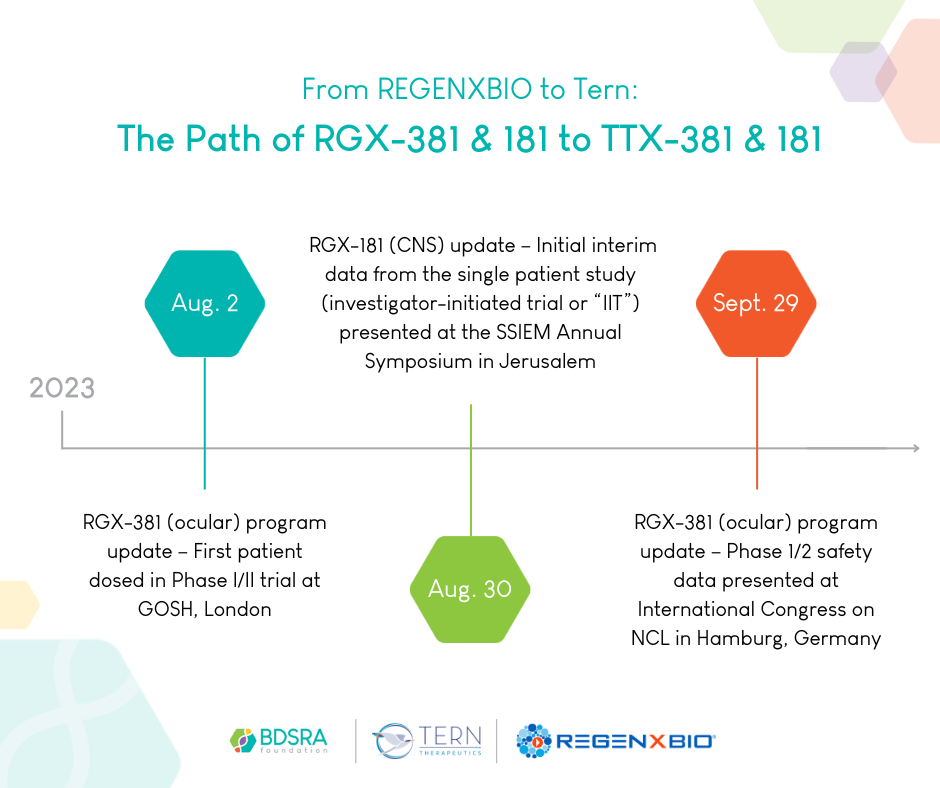
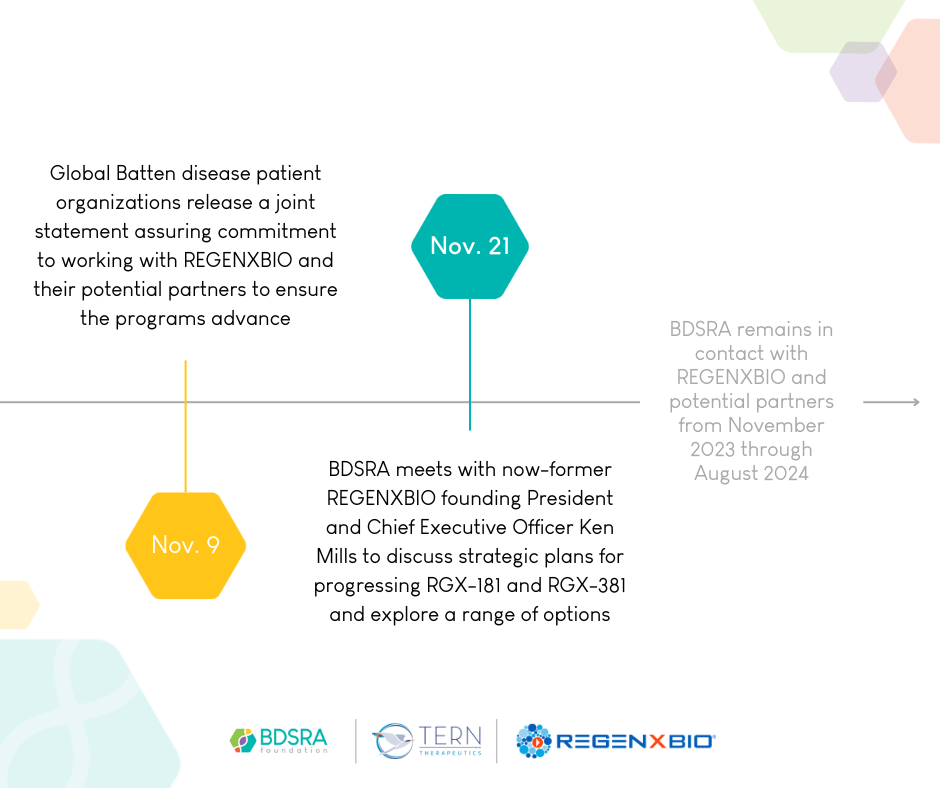
Timeline
A lot can change in one year, but advocating for our Batten community is something that won’t. Sometimes we cannot share updates from our industry partners, but our work behind the scenes never stops. Thank you for your patience and for trusting us to advocate for families of all CLN types.