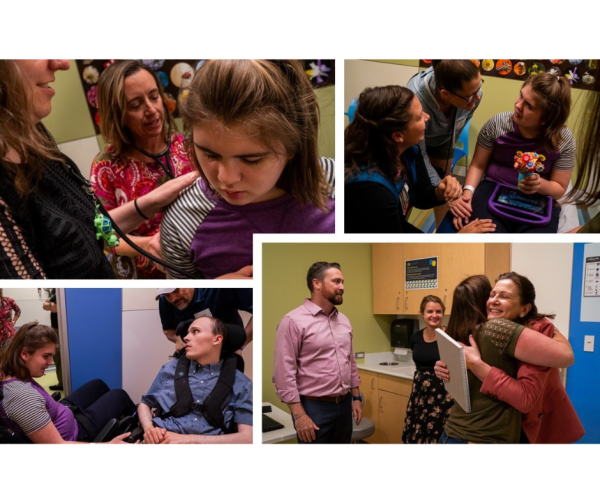It’s time for research updates! BDSRA Foundation’s Head of Research & Medical Affairs Dr. Ineka Whiteman breaks down Batten disease clinical program updates, research news & opportunities, and resources in her monthly column, as seen in BDSRA’s monthly newsletter, The Illuminator.
Centers of Excellence and Research Visits
During my recent visit to the U.S., coinciding with the Annual Family Conference in St. Louis, I was excited to have the opportunity to visit two U.S. Batten Disease Centers of Excellence, and the Pediatric Storage Disorders Lab led by Prof. Jon Cooper.
As part of my role as Program Lead for the Clinical Centers of Excellence Program, I am enjoying the opportunity to visit our Centers in person to meet the multidisciplinary teams and see firsthand how clinics operate in their management and care for Batten patients and families. In early July, I was invited to attend the Batten Disease Clinic at Children’s Hospital Colorado in Denver, led by Directors Dr. Kourtney Santucci and Dr. Scott Demarest. It was wonderful to observe their team in action with Batten patients being seen by neurology, clinical genetics, pediatrics, neuropsychology, psychiatry, speech pathology, occupational therapy, physical therapy, and other disciplines over 1-2 days as part of their 6-monthly visits.
Similarly, at the Children’s Hospital of Orange County (CHOC) in Los Angeles, I was invited to meet with the Batten Disease Clinic Director, Dr. Ray Wang, and the multidisciplinary team, and to present on the global prevalence of NCLs, international patient group initiatives and the Centers of Excellence program.
In St. Louis, I was honored to tour the laboratory of NCL research legend Prof. Jonathan Cooper PhD to meet his team and observe their neuropathology research in action. Prof. Cooper’s Pediatric Storage Disorders Lab is the leading international center for the morphological analysis of pathological changes in the central and peripheral nervous systems in NCL.
I look forward to more Centers of Excellence and research visits later this year.

Children’s Hospital Orange County (CHOC) Batten Disease Clinic and Neurology/Metabolic team members with Clinic Director Dr. Ray Wang (seated right in the photo above) and Dr. Ineka Whiteman (seated left in the photo above); and outside CHOC (below).

Dr Ineka Whiteman with Prof. Jon Cooper (2nd from left in the photo above) and the Pediatric Storage Disorders Lab team; and touring the research facilities in St. Louis (below).
Tern Therapeutics Launches with $15 Million Financing and Pipeline in CLN2 Batten Disease

We are heartened to share that on August 27, Tern Therapeutics announced its launch and acquisition of the investigational gene therapy programs RGX-381 and RGX-181 from REGENXBIO, for treating CLN2 disease.
In its press release, Tern announced it has entered into a global licensing agreement with REGENXBIO for both gene therapy products, now designated TTX-381 and TTX-181, respectively, to form its initial therapeutic pipeline. TTX-381 and TTX-181 are novel one-time gene therapy products being developed for the treatment of ocular and central nervous system (CNS) manifestations of CLN2 Batten disease, respectively. Tern also advised its closing of a $15 million funding round to accelerate ongoing clinical testing of TTX-381 and to advance its pipeline.
In a show of unity, REGENXBIO simultaneously released a letter to the Batten community expressing their support for these new developments and the team at Tern Therapeutics.
In recent years, we have seen highs and lows in the development of these CLN2 gene therapy programs, as outlined in the timeline below. Last November, REGENXBIO communicated they would not be moving forward with the development of RGX-381 and RGX-181 and would pursue strategic alternatives to continue advancing these programs. BDSRA Foundation, with our global patient advocacy partners, has worked diligently behind the scenes to support the process and ensure our CLN2 community has a consistent voice at the table.
We now look forward to continuing the collaboration with Tern to ensure these programs advance as swiftly and effectively as possible. It is encouraging to know the Tern management team brings deep expertise in gene therapy and ophthalmology and an established relationship with the Batten disease community. CEO and Co-Founder Alex Bailey, PhD, and Chief Medical Officer (CMO) and Co-Founder Christina Ohnsman, MD, were integral leaders in the CLN2 Batten disease programs at REGENXBIO. Indeed, the peer-reviewed paper OCT Biomarkers in Ocular CLN2 Disease in Patients Treated With Intraventricular Enzyme Replacement Therapy, was co-authored by Dr. Ohnsman and published this month. Read more in the Publication Highlights section below.
For more information on the announcement, check out Tuesday’s press release, visit the Tern Therapeutics website or contact patientadvocacy@terntx.com.
THE YEAR IN REVIEW: Timeline of events for the “381” & “181” CLN2 gene therapy programs
- August 2, 2023: RGX-381 (ocular) program update – First patient dosed in Phase I/II trial at Great Ormond Street Hospital (GOSH), London
- August 30, 2023: RGX-181 (CNS) update – Initial interim data from the single patient study (investigator-initiated trial or “IIT”) presented at the SSIEM Annual Symposium in Jerusalem
- September 29, 2023: RGX-381 (ocular) program update – Phase 1/2 safety data presented at International Congress on NCL in Hamburg Germany
- November 8, 2023: REGENXBIO releases its third-quarter earnings release, announcing a corporate restructuring that included the decision to halt the development of multiple investigational AAV gene therapies, including the RGX-181 and RGX-381 programs
- November 8, 2023: REGENXBIO releases a letter to the Batten community noting that the dosed patients will continue to be followed and that new patients will not be enrolled in the two trials. REGENXBIO also assures commitment to identifying a partner to advance the investigational therapies to patients
- November 9, 2023: Global Batten disease patient organizations release a joint statement assuring commitment to working with REGENXBIO and their potential partners to ensure the programs advance
- November 21, 2023: BDSRA meets with now-former REGENXBIO founding President and Chief Executive Officer Ken Mills to discuss strategic plans for progressing RGX-181 and RGX-381 and explore a range of options
- November 2023-August 2024: BDSRA remains in contact with REGENXBIO and potential partners
- February 7-8, 2024: Interim results for RGX-181 and RGX-381 presented at WORLDSymposium in San Diego, CA.
- April 9, 2024: Interim results for RGX-181 and RGX-381 presented during BDSRA Ask-An-Expert webinar.
- August 27, 2024: Tern Therapeutics announces acquisition of RGX-381 and RGX-181 (now designated TTX-381 and TTX-181, respectively) CLN2 gene therapy programs.
As always, thank you for your support and dedication to our shared mission.
Warm regards,

CLINICAL PROGRAM UPDATES
Clinical Trial Tracker
Keep up-to-date with the latest clinical trial and natural history study news with our Clinical Studies Chart on BDSRA’s website. Check it out by clicking here.
Neurogene Inc. – Gene Therapy for CLN5 Batten disease
Phase 1/2 clinical trial now fully enrolled
On Friday, August 9, Neurogene Inc. announced enrollment is now complete for its Phase 1/2 investigational trial of NGN-101 Gene Therapy for the treatment of CLN5 Batten disease, with a total of six patients enrolled (Clinical trial ID: NCT05228145). Neurogene unveiled this news in its Second Quarter 2024 Financial Results and Highlights Recent Updates.
According to the report, Neurogene plans to provide interim clinical data and a regulatory update in the first quarter of 2025. Read our global update here.
FAMILY REGISTER
Have you joined the Register yet?
The BDSRA Foundation Family Register is a vital tool that enables us to keep you informed of ongoing Batten disease research, including future clinical research and natural history opportunities.
The Register also enables BDSRA to understand more the prevalence of Batten disease, including the different subtypes and geographical locations. This helps us tailor our education and support activities according to the needs of our families. The Register is open to all current and bereaved families in the U.S. and internationally.
The information collected in this form is kept STRICTLY CONFIDENTIAL. Your involvement in this survey is entirely voluntary, and you may request to be removed from the list at any time. The form takes just a few minutes to complete and can be accessed by clicking here.
Thank you for participating in this important initiative!
PUBLICATION HIGHLIGHTS – August 2024
OCT Biomarkers in Ocular CLN2 Disease in Patients Treated With Intraventricular Enzyme Replacement Therapy
Huang WC, Ohnsman CM, et al. Invest Ophthalmol Vis Sci. 2024 Jul 1;65(8):45
ABSRACT
Purpose
Bilateral progressive, symmetrical loss of central retinal thickness (CRT) has been described in neuronal ceroid lipofuscinosis type 2 (CLN2) disease. This study details the pattern of morphological changes underlying CRT loss and disease progression in patients receiving intracerebroventricular (ICV) enzyme replacement therapy (ERT) with cerliponase alfa.
Methods
Spectral-domain optical coherence tomography macular cube scans were collected from 16 patients with classic CLN2 disease receiving ICV ERT. Detailed retinal structure analyses were performed on manually segmented horizontal B-scans through the fovea to determine the thickness of six retinal parameters and the extent of ellipsoid zone (EZ) loss.
Results
Anatomical changes primarily occurred in photoreceptor (PR)-related retinal parameters and correlated with ocular disease severity. Retinal degeneration began with initial focal parafoveal EZ discontinuities signaling the onset of rapid PR degeneration in a predictable pattern: parafoveal PR involvement with foveal sparing followed by profound parafoveal and foveal PR loss with additional thinning beyond the central retina. PR degeneration began with outer segment loss and progressed to outer nuclear layer (ONL) involvement. Longitudinal analyses confirmed these observations. The rate of PR loss was fastest at the fovea at ∼58 mm per year and became slower at locations farther away from the fovea.
Conclusions
Retinal degeneration in CLN2 disease is primarily associated with PR loss in a predictable pattern, with EZ disruption signaling early PR stress. CRT, ONL thickness, and PR layer thickness are useful anatomical biomarkers for understanding disease progression and treatment efficacy in CLN2. Studies using en face images will further clarify CLN2-related retinal degeneration.
Access the article here.
Ceroid lipofuscinosis type 2 disease: Effective presymptomatic therapy—Oldest case of a presymptomatic enzyme therapy.
Breuillard D, et al. Eur J Neurol. 2024 Sep;31(9):e16324. PMID: 38693756
ABSTRACT
Neuronal ceroid lipofuscinosis type 2 (CLN2) disease is a rare, lysosomal storage disorder that causes pediatric onset neurodegenerative disease. It is characterized by mutations in the TPP1 gene. Symptoms begin between 2 and 4 years of age with loss of previously acquired motor, cognitive, and language abilities. Cerliponase alfa, a recombinant human TPP1 enzyme, is the only approved therapy. We report the first presymptomatic cerliponase alfa intraventricular treatment in a familial case of CLN2 related to a classical TPP1 variant. Sister 1 presented with motor, cognitive, and language decline and progressive myoclonic epilepsy since the age of 3 years, evolved with severe diffuse encephalopathy, received no specific treatment, and died at 11 years. Sister 2 had a CLN2 presymptomatic diagnosis and has been treated with cerliponase since she was 12 months old. She is now 6 years 8 months and has no CLN2 symptom except one generalized seizure 1 year ago. No serious adverse event has occurred. Repeated Wechsler Preschool and Primary Scale of Intelligence, Fourth Edition standardized index scores are heterogeneous in the extremely low to low average ranges. Mean length of utterances, a global index of sentence complexity, showed a delay, but a gradual improvement. The reported case enhances the major contribution of presymptomatic diagnosis and significant middle‐term treatment benefit for patients with CLN2.
Access the article here.